Process of Putting Monomers Together Is Called
Up to 24 cash back 7. Oxygen into hydrogen store water or h2o.

Synthesis Of Biological Macromolecules Boundless Biology
Condensation also called this refers to the removal of a water molecule during the linking of monomers.
. Monomers Polymers and Dehydration Synthesis. What differentiates one amino acid from another. The significance of directionality of the monomers in a polymer is that when you put the monomers together in a certain sequenceorder they have a.
The monomer molecules may be all alike or they may represent. Carbohydrate monomers are 10. Polymerization any process in which relatively small molecules called monomers combine chemically to produce a very large chainlike or network molecule called a polymer.
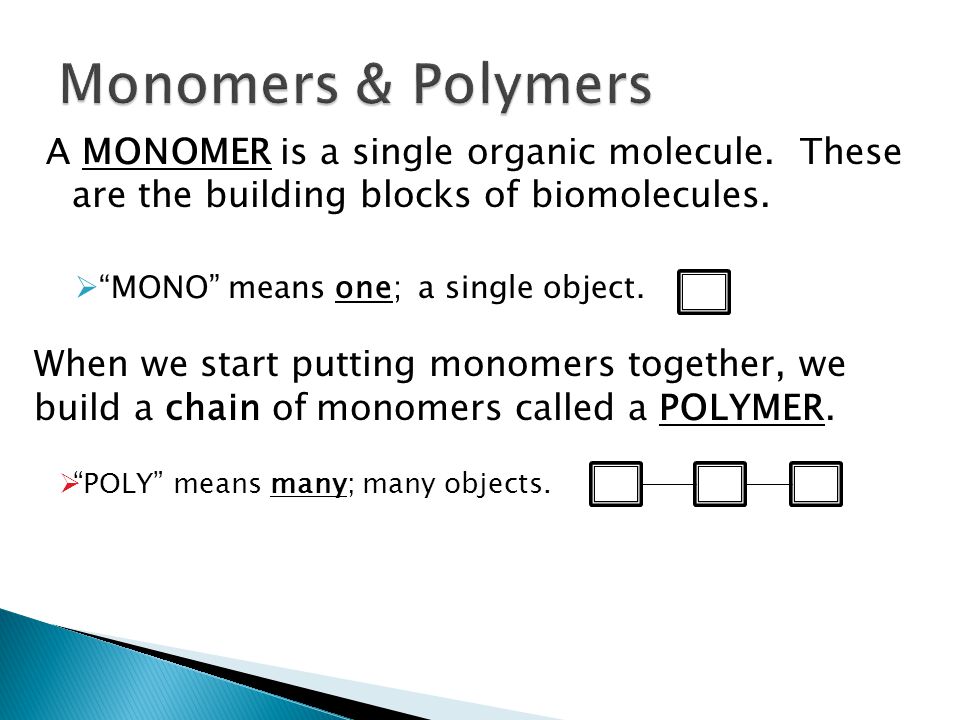
The process of putting monomers together is called. Different structures and functions. This type of reaction is known as dehydration synthesis w.
Polymerization any process in which relatively small molecules called monomers combine chemically to produce a very large chainlike or network molecule called a polymer. What must be added to break the bonds. This is the opposite of a condensation reaction.
The name of the process during which a bond between two monomers is broken. What is the process of putting monomers together. The process of putting monomers together is.
What is lost during the process of 11. The-significance of directionality of the monomers in a polymer is that when you put the monomers together in a certain sequence order they have a. What is the process by which cells link monomers together.
The significance of directionality of the monomers in a polymer is that when you put the monomers together in a certain sequenceorder they have Different structures and functions. Facts from worksheets Learn with flashcards games and more for free. The process for connecting two monomers together forming a covalent bond is called dehydration synthesis.
Others are large and unwieldy and can contain hundreds or thousands of atoms. Another name for the condensation reaction. The significance of directionality of the monomers in a polymer is that when you put the monomers together in a certain sequenceorder they have.
The significance of directionality of the monomers in a polymer is that when you put the monomers together in a certain sequenceorder they have. An H is added to one monomer and an OH is added to the other. Different functions and structures.
What kind of bond is formed in early specifically between amino acids of a protein. View the full answer. The process of splitting the bond between monomers is called hydrolysis.
What kind of bond is. What differentiates one amino acid from another. The monomer molecules may be all alike or they may represent two three or more different compounds.
Ans 1- Dehydration synthesis The monomers combine with each other using covalent bonds to form larger molecules known as polymers. The significance of directionality of the monomers in a polymer is that when you put the monomers together in a certain sequenceorder they have a. Hydrolysis means to break with water.
The process of putting monomers together is called b. This process is called polymerization. Usually at least 100 monomer molecules must be combined to make a product that has certain.
In doing so monomers release water molecules as byproducts. The process of putting monomers together is called. Biological Molecules BIOL1107 Fall 2014 Depending on which directions they have different structures and functions 11.
During this process the indivudual monomers give off a gas which enables them to form a macromolecule. What is lost during the process of 11. Some biological molecules are relatively small and may contain a handful of atoms bound together.
Carbohydrate monomers are 10. If you were trying to correctly assemble a molecule that big you would probably want to start by putting together some smaller fragments and then. Monomers group together to form a macromolecule during a process known as polymerization.
Up to 24 cash back 7. The process of putting monomers together is called meaning. What is the process of putting monomers together.
This type of reaction is dehydration synthesis which means to put together while losing water How does the bond between monomers are broken down. In doing so monomers release water molecules as byproducts. There are many forms of polymerization and different systems exist to categorize them.
The process of putting monomers together is called b. The process of putting monomers together is called. The process of 1 putting monomers together is called 11 rJ k v c.
Made out of monomers. The significance of directionality of the monomers in a polymer is that when you put the monomers together in a certain sequenceorder they have _____ 11. In polymer chemistry polymerization American English or polymerisation British English is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks.

Synthesis Of Biological Macromolecules Boundless Biology

No comments for "Process of Putting Monomers Together Is Called"
Post a Comment